On December 2, our website will be updated as we continue to focus on delivering a digital experience that is easy, enjoyable and effective. The site will undergo maintenance from 8 a.m. – 3 p.m. ET as we make these improvements. We apologize for any inconvenience.
Deliver To:
- Afghanistan
- Albania
- Algeria
- Amer.Virgin Is.
- American Samoa
- Andorra
- Angola
- Anguilla
- Antarctica
- Antigua Barbuda
- Argentina
- Armenia
- Aruba
- Australia
- Austria
- Azerbaijan
- Bahamas
- Bahrain
- Bangladesh
- Barbados
- Belarus
- Belgium
- Belize
- Benin
- Bermuda
- Bhutan
- Bolivia
- Bonaire, Saba
- Bosnia-Herz.
- Botswana
- Bouvet Island
- Brazil
- Brit.Ind.Oc.Ter
- Brit.Virgin Is.
- Brunei Daruss.
- Bulgaria
- Burkina Faso
- Burundi
- C. African Rep.
- Cambodia
- Cameroon
- Canada
- Cape Verde
- Cayman Islands
- Chad
- Chile
- Christmas Islnd
- Coconut Islands
- Colombia
- Comoros
- Congo
- Cook Islands
- Costa Rica
- Cote d'Ivoire
- Croatia
- Curacao
- Cyprus
- Czech Republic
- Dem. Rep. Congo
- Denmark
- Djibouti
- Dominica
- Dominican Rep.
- Ecuador
- Egypt
- El Salvador
- Equatorial Guin
- Eritrea
- Estonia
- Ethiopia
- Falkland Islnds
- Faroe Islands
- Fiji
- Finland
- France
- Frenc.Polynesia
- French Guiana
- French S.Territ
- Gabon
- Gambia
- Georgia
- Germany
- Ghana
- Gibraltar
- Greater China (Chinese Mainland)
- Greater China (Hong Kong SAR)
- Greater China (Macau SAR)
- Greater China (Taiwan)
- Greece
- Greenland
- Grenada
- Guadeloupe
- Guam
- Guatemala
- Guinea
- Guinea-Bissau
- Guyana
- Haiti
- Heard McDon.Isl
- Honduras
- Hungary
- Iceland
- India
- Indonesia
- Iraq
- Ireland
- Israel
- Italy
- Jamaica
- Japan
- Jordan
- Kazakhstan
- Kenya
- Kiribati
- Kosovo
- Kuwait
- Kyrgyzstan
- Laos
- Latvia
- Lebanon
- Lesotho
- Liberia
- Liechtenstein
- Lithuania
- Luxembourg
- Madagascar
- Malawi
- Malaysia
- Maldives
- Mali
- Malta
- Marshall Islnds
- Martinique
- Mauritania
- Mauritius
- Mayotte
- Mexico
- Micronesia
- Minor Outl.Isl.
- Moldova
- Monaco
- Mongolia
- Montenegro
- Montserrat
- Morocco
- Mozambique
- Myanmar
- N.Mariana Islnd
- Namibia
- Nauru
- Nepal
- Netherlands
- New Caledonia
- New Zealand
- Nicaragua
- Niger
- Nigeria
- Niue
- Norfolk Island
- North Macedonia
- Norway
- Oman
- Pakistan
- Palau
- Panama
- Pap. New Guinea
- Paraguay
- Peru
- Philippines
- Pitcairn
- Poland
- Portugal
- Puerto Rico
- Qatar
- Reunion
- Romania
- Russian Fed.
- Rwanda
- S. Sandwich Ins
- S.Tome,Principe
- Saint Helena
- Saint Lucia
- Samoa
- San Marino
- Saudi Arabia
- Senegal
- Serbia
- Seychelles
- Sierra Leone
- Singapore
- Sint Maarten
- Slovakia
- Slovenia
- Solomon Islands
- Somalia
- South Africa
- South Korea
- Spain
- Sri Lanka
- St Kitts&Nevis
- St. Vincent
- St.Pier,Miquel.
- Suriname
- Svalbard
- Swaziland
- Sweden
- Switzerland
- Tajikistan
- Tanzania
- Thailand
- Timor-Leste
- Togo
- Tokelau
- Tonga
- Trinidad,Tobago
- Tunisia
- Turkey
- Turkmenistan
- Turksh Caicosin
- Tuvalu
- Uganda
- Ukraine
- United Kingdom
- United States
- Uruguay
- Utd.Arab Emir.
- Uzbekistan
- Vanuatu
- Vatican City
- Venezuela
- Vietnam
- Wallis,Futuna
- West Sahara
- Yemen
- Zambia
- Zimbabwe

12 innovations recognized for Edison Best New Product Awards™
For the 2nd year in a row, we've set a record for the highest number of Edison Awards finalists in a single year. It's also the 7th consecutive year that Dow has had more finalists than any other organization.

Explore Markets

6 Dow products win 2024 BIG Innovation Awards
Dow brands DOWSIL™, EcoSense™, ELECPURE™, RHOBARR™, SYL-OFF™ were recognized in the chemical and manufacturing sub-categories for bringing new ideas to life in innovative ways.

Introducing Sustainability Science
New brands from Dow make it easier for customers to find and purchase our products with sustainability benefits – while adhering to high standards of transparency and claim substantiation.

Discover the many benefits Dow.com has to offer
Welcome to our newly updated website aimed at delivering a digital experience that is easy, enjoyable and effective – for you! Click below to watch a quick video showcasing our new site.
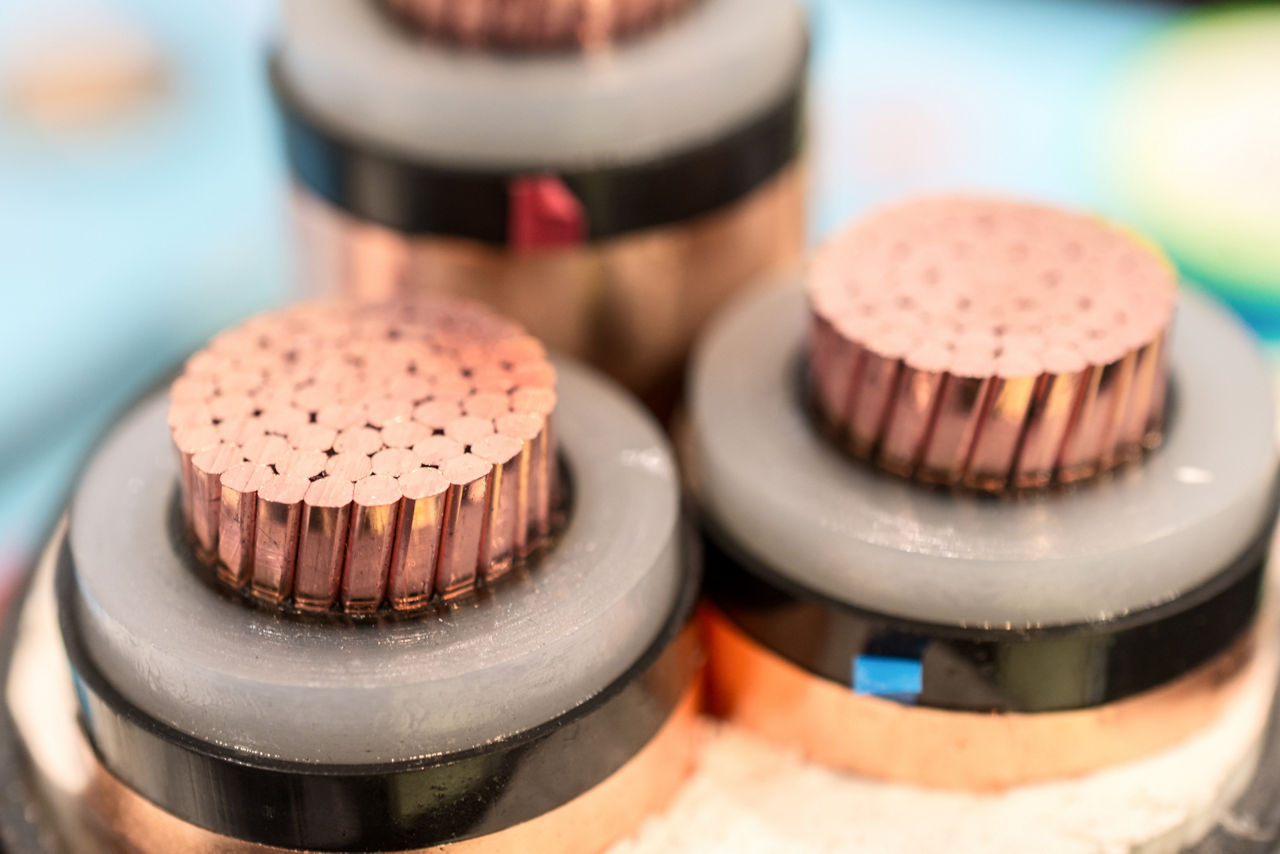
The future of cable manufacturing
Learn how ENDURANCE™ compounds for power cable systems can increase manufacturing efficiency with added sustainability benefits.

Driving more sustainable mobility
Through collaboration and research, our MobilityScience™ team helps advance the mobility industry with innovative, low-carbon solutions.